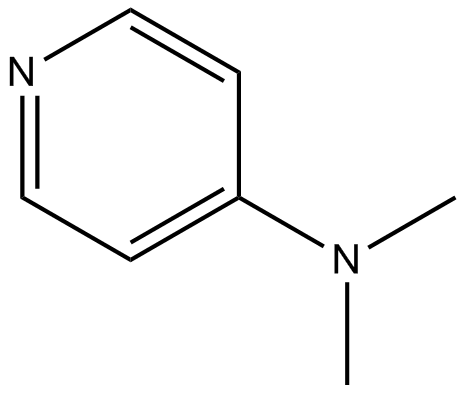
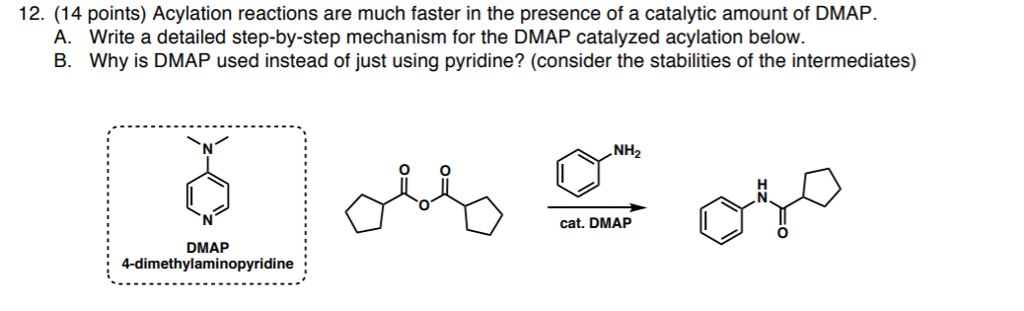
Dmap Reagent Mechanism. Simple Method for the Esterification of Carboxylic Acids DMAP is a very effective acyl transfer agent. Because of its basicity, DMAP is a useful nucleophilic catalyst for a variety of reactions such as esterifications with anhydrides, the Baylis-Hillman reaction, hydrosilylations, tritylation, the Steglich rearrangement, Staudinger synthesis of β-lactams and many more. This highly electrophilic agent is then attacked by the alcohol to form the product ester. Connors and Albert recommend using acetic anhydride/- DMAP as a reagent for the quantitiative determination of hydroxyl groups in alcohols and phenols. Common Uses: Nucleophilic catalyst for Boc protections. Reagent for amide couplings ( EDC + DMAP) DMAP accelerates the reaction rate by reacting with the O -acylisourea intermediate to form a highly activated electrophilic acylated pyridinium intermediate. DMAP acts as an acyl transfer reagent in this way, and subsequent reaction with the alcohol gives the ester. A protocol for amide coupling by in situ formation of acyl fluorides and reaction with amines at elevated temperature has been developed and found to be efficient for coupling of sterically hindered substrates and electron deficient amines where standard methods failed.

Dmap Reagent Mechanism. Because of its basicity, DMAP is a useful nucleophilic catalyst for a variety of reactions such as esterifications with anhydrides, the Baylis-Hillman reaction, hydrosilylations, tritylation, the Steglich rearrangement, Staudinger synthesis of β-lactams and many more. Addition of the carboxylate to the carboxylic acid chloride forms the mixed anhydride: DMAP is an acyl transfer reagent that reacts regioselectively at the less hindered carbonyl site: DMAP is a stronger nucleophile than the alcohol. This highly electrophilic agent is then attacked by the alcohol to form the product ester. A protocol for amide coupling by in situ formation of acyl fluorides and reaction with amines at elevated temperature has been developed and found to be efficient for coupling of sterically hindered substrates and electron deficient amines where standard methods failed. This intermediate cannot form intramolecular side products but reacts rapidly with alcohols. Dmap Reagent Mechanism.
Connors and Albert recommend using acetic anhydride/- DMAP as a reagent for the quantitiative determination of hydroxyl groups in alcohols and phenols.
Performed in dichloromethane at room temperature, this reaction is readily tolerated by a broad scope of substrates, yielding alkenes preferentially with the (E)-geometry.
Dmap Reagent Mechanism. Reaction mechanism [ edit] The aliphatic carboxylate adds to the carbonyl carbon of Yamaguchi reagent, forming a mixed anhydride, which is then attacked by DMAP regioselectively at the less hindered carbon, producing acyl-substituted DMAP. To overcome the limitation of connectivity maps on data coverage, we constructed a comprehensive in silico drug-protein connectivity map called DMAP, which contains directed drug-to-protein effects and effect scores. A protocol for amide coupling by in situ formation of acyl fluorides and reaction with amines at elevated temperature has been developed and found to be efficient for coupling of sterically hindered substrates and electron deficient amines where standard methods failed. Addition of the carboxylate to the carboxylic acid chloride forms the mixed anhydride: DMAP is an acyl transfer reagent that reacts regioselectively at the less hindered carbonyl site: DMAP is a stronger nucleophile than the alcohol. The utility of triphosgene and DMAP as mild reagents for chemoselective dehydration of tertiary alcohols is reported.
Dmap Reagent Mechanism.